Expert report

DR. JOSEPH MONSONEGO
- Director of Institut du Col, Paris
- President of EUROGIN (European Research Organization on Genital Infection and Neoplasia)
www.eurogin.com - President of the International Foundation WACC (Women Against Cervical Cancer)
www.wacc-network.org
Of the 15 types of HPV considered to be high-risk for cervical cancer, five (16, 18, 33, 31 and 45) are linked to the highest risk, with types 16 and 18 causing 60% of CIN3 and 70% of cancers. The natural history of the disease must therefore be reconsidered in its entirety, taking into account high-risk exposure to the most active types of the virus2.
Peak prevalence of the infection is observed at around 20 to 25 years of age, where it can reach 30%; prevalence gradually decreases with age and averages an estimated 10-12% in people over 304. Young women are at a higher risk owing to insufficient preparation of the immune system, immaturity of the metaplastic epithelium in younger women and increased exposure to the virus through higher numbers of sexual partners.
Instantaneous infection is transient and short-lived in almost 90% of cases and clears within 18 to 36 months, especially those associated with the less active viruses5. However, 10% of infections are persistent, more often those found to be associated with the most active viruses. This clearance of the virus can be attributed to natural immunity, the specific mechanisms of which have not been fully explained. Nevertheless, natural immunity is not enough to ensure definitive protection and it is possible to become infected with the same virus again or with others6. This is due to the fact that HPV infection is not as immunising as vaccination is effective.
These factors have also been found to be risk markers for cervical cancer.
Nicotine and its carcinogenic metabolites are found in the cervix mucous membrane and smoking has an impact on local immune markers. Oestrogen and progesterone may have an effect on cell proliferation and thus induce susceptibility to DNA damage.
Cervical
carcinogenesis
Cervical carcinogenesis always begins as a result of exposure to HPV, particularly to the 5 most active viruses. Viral persistence leads to morphological changes in the cells, some of which, particularly Low Grade lesions (LSIL), may then regress or disappear as natural adaptive immunity is gradually established. High Grade lesions (HSIL) are considered to be high-risk lesions with the potential to progress. Observational data shows that the time in which the persistent virus may lead to cancer after exposure of the cervical transformation zone may be estimated at between 15 and 20 years, although faster progression may have been reported.
The concept of a continuum from LSIL to HSIL is no longer commonly accepted. The specific mechanisms involved in regression and progression, which are most certainly immunological and viral in nature, are still not fully understood. The initial immune response to acute infections is probably mediated by the local innate immune system, and is likely to involve mechanisms such as the activation of toll-like receptors and natural killer cells. Persistent infections probably involve adaptive immune responses, which rely on antigen-presenting cells12. HPV 16 is controlled by both innate immunity and adaptive immunity.
and persistence
of the HPV
These oncoproteins disrupt the cell cycle by interfering with negative cell cycle regulators (p53 and pRB), thus causing the cell to become unstable, the starting point of the transformation. While the virus reaches maturation in LSILs, in HSILs it is in what is known as an episomal state (immature), and is incorporated into the cell genome in cancers13.
MICROBIOTA AND HPV
The microbiota plays an important role in human health.
It has been reported that an imbalance in the microbiota may be involved in colon cancer and atopic dermatitis. The vaginal microbiota is made up of a large variety of Lactobacilli, the balance and diversity of which can have an effect on the local immune state and vice versa14.
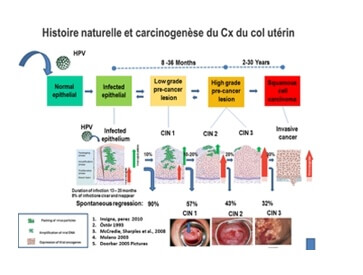
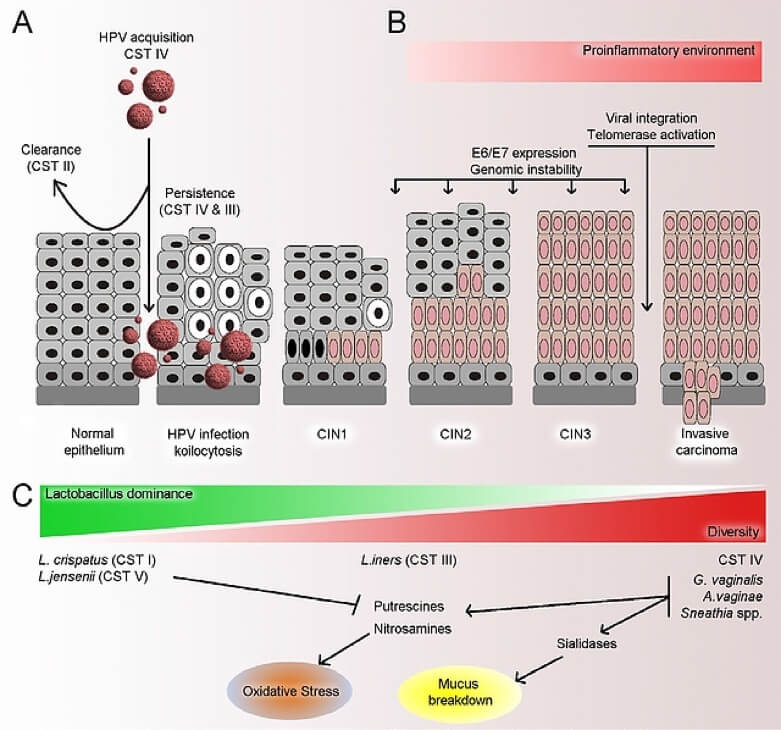
Figure 2. Adapted from Mitra et al. Microbiome (2016).
In vitro studies show that certain Lactobacilli (Lactobacillus spp) exert cytotoxic effects on cervical tumour cells15]. This indicates that cervical cells, the microbiota and its metabolites are likely to interact15.
There is substantial evidence to suggest that HPV is affected by the vaginal microbiota (VM). In meta-analyses of most cross-sectional studies, the presence of bacterial vaginosis (BV) was linked to higher rates of HPV infection (12 studies: odds ratio [OR]): 1.43, CI 95%: 1.11-1.84)16. Lactobacilli-depleted microbiota could contribute to HPV persistence.
Other work indicates that HPV persistence is more likely in people with an altered microbiota. One study showed that women exhibiting HPV persistence had a BV prevalence of 11% compared to only 5% in women who had cleared their HPV17. In addition, King et al.18 found that women with bacterial vaginosis exhibited a delayed viral clearance (adjusted hazard ratio 5 0.84, 95% CI: 0.72, 0.97).
One of the first studies aimed at examining the link between the vaginal microbiota (VM) and HPV was conducted in Korea and used cervico-vaginal samples collected from 912 women19. Out of 78 women 68 were analysed, 23 of whom were HPV-positive and 45 of whom were HPV-negative. An analysis of 45 premenopausal women with or without an HPV infection showed that certain bacteria (Fusobacteria, particularly Sneathia spp) could be used as microbiological markers for HPV infection. The HPV-positive women exhibited greater microbiota diversity and a lower proportion of Lactobacillus spp than the HPV-negative women.
Other data show that the microbiota plays an important role in modulating the immune system of the female genital tract20.
A number of questions must be answered as part of the work in progress:
- Does the microbiota observed during the immature squamous metaplasia in young women explain why HPV infection is more likely at a young age?
- Do risk factors for HPV persistence (smoking, immunodepression, oral contraception) have a negative influence on the microbiota by helping to advance the carcinogenesis process?
- Is the age-related difference in prevalence of HPV, HSIL and cervical cancer a result of the changes in microbiota diversity with age?
- Do HPV-induced variations in the microbiota trigger oncogenic changes at the time of carcinogenesis?
Conclusion
In conclusion, the vaginal microbiota appears to play a role in the acquisition and persistence of HPV and in the development and progression of CIN. There is a need for further studies to prove that these outcomes are influenced by the composition and modified diversity of the microbiota 15.
These data may provide opportunities to develop novel therapeutic agents that aim to restore or preserve the vaginal microbiota, such as specific prebiotics (bioselective substrate for the growth of beneficial Lactobacillus, to the detriment of pathogens)21,22, in order to prevent HPV infection and, in infected women, limit the risk of precancerous lesions.

DR. JAVIER CORTÉS BORDOY
- Senior Consultant in Gynecology Oncologic.
- Private practice.
- Palma de Mallorca.
- EUROGIN 2015 President.
- Ex-president of the Spanish Association of Cervical Pathology and Colposcopy.
PRESENCE OF THE HUMAN
PAPILLOMAVIRUS (HPV)
In the CLEOPATRE1 study, we found that in Spain the average rate of HPV in women between 18 and 65 years old was 14%, shown a stable curve at the level of 30% in the age group 18-30 years, and a fast decrease to another level maintained around 10% for 30 to 35 year old.
Consequently, it can be said that in the first third of life in Spain, the presence of HPV in cervical mucus is very common. For more and more well-understood reasons (type of HPV, host immunity, among others) generally, there is a clearance of this viral presence, except for a group of women in which it has not taken place. A group that can be labeled as chronic carriers of the HPV. This is a situation that we consider of high risk for the initial development of precancerous lesions of the cervix, which leads to a high percentage, of invasive cervical cancer 2.
High risk does not mean absolute certainty. It can be deduced, from the estimated cervical precancerous lesions and documented invasive cervical cancer rates, that only a limited proportion of women with HPVn will eventually – if unchecked – develop cervical lesions 3. Why?
PATHOGENESIS OF HPV INFECTION
Above other basic considerations related to the biology of the process, the answer to this question has recently been added to our knowledge. Although it is very simple: keep in mind HPV in cervical or anal mucus does not stricktly mean to be infected by HPV.
Being infected represents a step further to the viral presence, it means that the HPV has been integrated into the cervical epithelium and has colonized cells.
For this reason, there is growing evidence of the usefulness of techniques that measure viral mRNA and expression of their oncogenes E6 and E7, because this expression is a marker of the integration of the virus into the cell genome and, consequently, the first step in the oncogenic process is made.
The cervix has a very unstable histological structure, with the permanent confrontation of two epithelia, polystratified vaginal squamous and endocervical cylindrical glandular (Figure 1). Below this glandular epithelium, the cell line is composed of «reserve» cells. These retain the ability to grow and differentiate more often into mature forms of squamous epithelium or glandular epithelium.
This process has been called metaplasia and creates a more or less extensive area identified as «transformation zone»5 in the cervix (Figure 1).
This is a normal process in sexually active women, increasing in users of hormonal contraception, IUDs or had children.
The HPV needs to integrate cells in mitotic activity. The reserve cells in metaplasic re-epithelization process fulfill this condition and therefore are ideal targets for anchoring HPV.
Consequently, well epithelized cervix with squamous epithelium, with limited extension or nonexistent areas of transformation would provide little suitable enviroment for inclusive colonization – and therefore oncogenic potential – for HPV.
PAPILOCARE® POTENTIAL PREVENTIVE STRATEGY
We have consistent evidence that the vaginal gel Papilocare® significantly improves re-epithelization of the cervix (95% of cases)7. We hypothesize that, through this implementation, this gel could limit, if not prevent, HPV integration, by reducing the epithelial conflict area with intense cellular activity that represents the perfect target for HPV integration. If the cervix is well epithelized, the possibility of HPV viral anchor with oncogenicity potential decreases significantly. This offers a new preventive strategy; easy to use, without appreciable side effects 7 for primary prevention of dependent HPV lesions.
Conclusion
Taking into account the above and understanding that the research should be continued, the “barrier effect” produced by Papilocare® allows contemplating the possibility of using it in patients with alterations of cervical epithelization to hinder the integration of HPV and prevent infection.
(FALTAM REFERÊNCIAS 4 E 6 NO TEXTO)
FALTAM REFERÊNCIAS 4 E 6 NO TEXTO
1. Castellsagué X, Iftner T, Roura E, Vidart JA, Kjaer SK, Bosch FX et al. Prevalence and genotype distribution of human papillomavirus infection of the cervix in Spain: the CLEOPATRE study. J Med Virol. 2012;84:947-56. 2. Moscicki AB, Schiffman M, Burchell A, Albero G, Giuliano AR, Goodman MT et al. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine. 2012; 30Suppl 5:F24-33. 3. Torné A, del Pino M, Cusidó M, Alameda F, Andia D, Castellsagué X et al. Guía de Cribado del Cáncer de Cuello de Útero en España, 2014. Prog Obstet Ginecol 2014; 57(Supl. 1):1-53. 4. Reid JL, Wright TC Jr, Stoler MH, Cuzick J, Castle PE, Dockter J. et al. Human Papillomavirus Oncogenic mRNA Testing for Cervical Cancer Screening: Baseline and Longitudinal Results From the CLEAR Study. Am J Clin Pathol. 2015; 144:473-83. 5. Curso de Colposcopia de la Asociación Española de Patología Cervical y Colposcopia. Disponible en http://www.aepcc.org/ Acceso: 07/09/15. 6. Egawa N, Egawa K, Griffin H and Doorbar J. Human Papillomaviruses: Epithelial Tropisms and the Development of Neoplasia. Viruses. 2015; 7:3863-90. 7. Palacios S.: Pilot study to evaluate the effect of a Coriolus versicolor based vaginal gel on the epithelialization of the cervix lesions. Poster. 30th International Papillomavirus Conference. Septiembre 17-21, 2015. Lisboa. Portugal.